Throughout the pandemic, many vaccine hesitant individuals have said they’re afraid of the jab because it’s been ‘rushed’ through approval processes. So what are Australia’s approval mechanisms for vaccines?
Vaccines have to go through four stages with the Therapeutic Goods Administration (TGA) before being authorised for distribution:
1. Pre-application:
The vaccine is assessed against specific eligibility criteria, such as the nature of preliminary clinical data, evidence of plans to submit comprehensive clinical data, and clinical need. If the applicant is successful, the vaccine will be granted provisional determination.
2. Application:
Once provisional application is granted, the applicant is then free to apply for provisional registration. This next step requires the submission of a comprehensive report including details of clinical studies, non-clinical/toxicology studies, information on the chemistry and manufacturing of the vaccine, and risk management.
3. Evaluation
Formal evaluation is undertaken after the application is approved. Technical experts oversee multiple phases of evaluation, working with the Advisory Committee on Vaccines, an independent committee of external experts.
4. Decision
The TGA will make a decision to approve a new vaccine on the basis of a benefit-risk analysis that assesses the vaccine’s safety, quality and effectiveness.
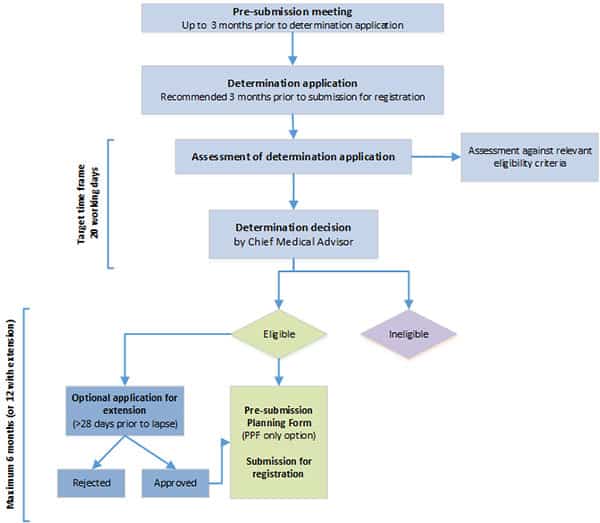
After it’s approved by the TGA, the vaccine will be included in the Australian Register of Therapeutic Goods as a provisionally registered medicine, a status that lasts for two years. This is what has now been granted to the Pfizer vaccine for 5-11 year olds. There’s an option to then apply for extensions of provisional registration up to a maximum of six years.
Even once the vaccine is approved and registered on the ARTGA, the TGA will continue to monitor it. The monitoring process is rigorous and “can rapidly detect investigate and respond to any emerging safety issues”.
Analysts closely track reports of adverse events, review medical literature and other new safety information, and collaborate with international regulators to ensure continued safety.
Safety reports are released weekly and easily accessible here. For example, the latest report was released on December 2nd, and includes risk analysis for rare cases of myocarditis, thrombosis, and other reported side-effects of COVID vaccines.
Unlike other countries – like the US, UK and Canada – Australia does not have an ‘Emergency Use Authorisation‘ process for COVID vaccines, meaning no part of the process was ‘rushed’. The only reason the rollout of COVID vaccines has been faster than normal is the increased funding and urgency around their development.
As with all vaccines being distributed and used in Australia, Pfizer, AstraZeneca and Moderna have undergone exhaustive screening with internal and external medical advisors. Not only have they have been determined safe and effective for all the age groups now authorised to access them, but their registration means the TGA has found them to be crucial in preventing threats to life or health posed by COVID-19.
Follow Maddie’s journalism journey on Twitter.
Cover photo by Hakan Nural on Unsplash.
Sign Up To Our Free Newsletter