Federal Health Minister Greg Hunt has announced Australia’s procurement of two new drugs used to treat COVID. 15,000 doses of Ronapreve and half a million courses of Pfizer’s treatment pill PF-07321332 are set to land on our shores and be put into use over the next year. This will add to existing treatment drug deals made over the past month.
Ronapreve
For some, the name Ronapreve may ring faint bells – it first made headlines in October 2020 when Donald Trump was treated with it. It’s already been licensed for emergency use in more than twenty countries, including the UK, US, EU, Japan and India.
Ronapreve is unusual in the world of infectious disease treatment because it uses monoclonal antibodies, a type of medicine that has up to now been used largely for cancer and autoimmune disorder treatment.
Clinical trials have yielded strong successes – in one trial hospitalisation was reduced by 70%, and symptom duration by four days. In another trial, individuals exposed to COVID and given the drug showed an 81% reduced likelihood of contracting COVID.
PF-07321332
Pfizer’s drug is the first orally administered experimental treatment to be assessed in clinical trials to specifically target COVID. It was granted provisional determination by the TGA earlier this month.
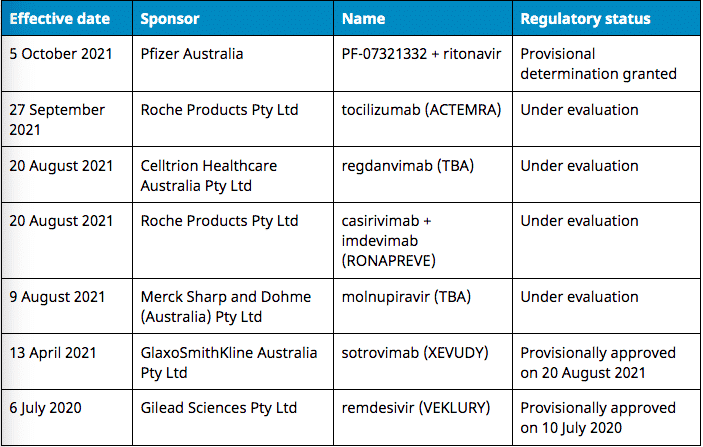
PF-07321332 is taken twice a day for a five-day course, and prevents the virus from replicating. It will be co-administered along with ritonavir, in order to slow the metabolism of the treatment and thus leave it active in the body for longer.
Sotrovimab
Sunday’s deal follows the earlier purchase of thousands of doses of sotrovimab, an intravenous COVID treatment. Just over seven thousand doses of the drug were initially secured in August, prior to sotrovimab’s approval by the TGA.
The order was later increased to 31,000 doses. Having received TGA approval on August 20th, half of these doses arrived at the beginning of October.
A single dose of sotrovimab reduces the likelihood of hospitalisation or death by almost 80%. The government estimates between 8-15% of people who are infected will be recommended for sotrovimab treatment.
Molnupiravir
Australia also purchased 300,000 courses of Merck’s oral treatment Molnupiravir in early October. The drug is another oral antiviral medication, but has not yet received emergency or formal approval in any country. The TGA has granted it provisional determination.

But Molnupiravir’s interim results were so strong that outside monitors recommended a premature stop to Merck’s phase 3 trial for it. All patients in the 775-person trial had at least one risk factor with poor disease outcome, such as obesity or older age.
Those that were given a placebo drug were hospitalized at twice the rate of those that took Molnupiravir. There were eight placebo deaths, but zero among those who took the drug. The Australian government anticipates approval for the drug could be granted as soon as early 2022.
Merck has also stated it will allow Molnupiravir to be sold by generic manufacturers in India at a significantly reduced price in over one hundred poorer countries, though no distribution agreements have been reached yet.
Treatment drugs in general are crucial as Australia starts to open up again, both domestically and internationally, to deal with the expected case surge. But oral drugs like PF-07321332 and Molnupiravir are revolutionary in their potential to greatly ease the strain on the hospital system.
Still, doctors like Dr Peter Hotez warn that “we have to be careful about saying that this is going to be a miracle. It’s still no substitute for vaccinating the world.”
Cover image by Ksenia Yakovleva on Unsplash.
Follow Maddie’s journalism journey on Twitter.