MDMA – commonly known as Ecstasy or Molly – could be approved in the US for treatment of post-traumatic stress disorder as early as next year. Phase 3 trials are currently underway, and if successful, will clear the way for FDA approval.
Scientists are excited about the huge step this represents for therapeutic psychedelics research. Many believe this approval could be the first of similar drug-assisted treatments for various other mental health issues.
MDMA’s fraught history
MDMA took off in psychotherapeutic circles in the 1970s, with hundreds of therapists administering it over the next two decades. They reported astounding results. Patients were making progress that would normally take years within just a few sessions.
Unfortunately, once Ecstasy escaped onto the streets and became a staple party drug, public opinion soured.
In 1985, the US DEA classified it as a Schedule I drug, making its possession an offence punishable by 15 years in prison. Propaganda posters appeared, with colourful ‘Swiss cheese’-esque brain scans, warning “This is your brain on ecstasy.”

Neurologists like George Ricaurte claimed MDMA was neurotoxic and would cause “permanent brain damage” with just one dose. Such claims were later debunked as applying to amphetamine, not MDMA.
Current Trials
Contemporary animal studies have revealed MDMA causes massive and long-lasting surges of serotonin. Serotonin is a mood stabiliser that controls happiness. These serotonin surges also trigger hormones that promote feelings of interpersonal closeness and bonding.
MDMA also reopens a stage of brain development neurologists call the ‘critical period’. This describes a phase when the brain is better able to make and store new memories. For sufferers of PTSD, returning to this malleable state allows them to process trauma related to negative memories.
In October of last year, the Multidisciplinary Association for Psychedelic Studies (MAPS) published its findings from the first ever late-stage psychedelic clinical trial. This first Phase 3 trial observed 90 participants, who included veterans, sexual assault and mass shooting survivors and first responders.
All participants had been diagnosed with PTSD for an average of over 14 years. 90% had considered suicide.
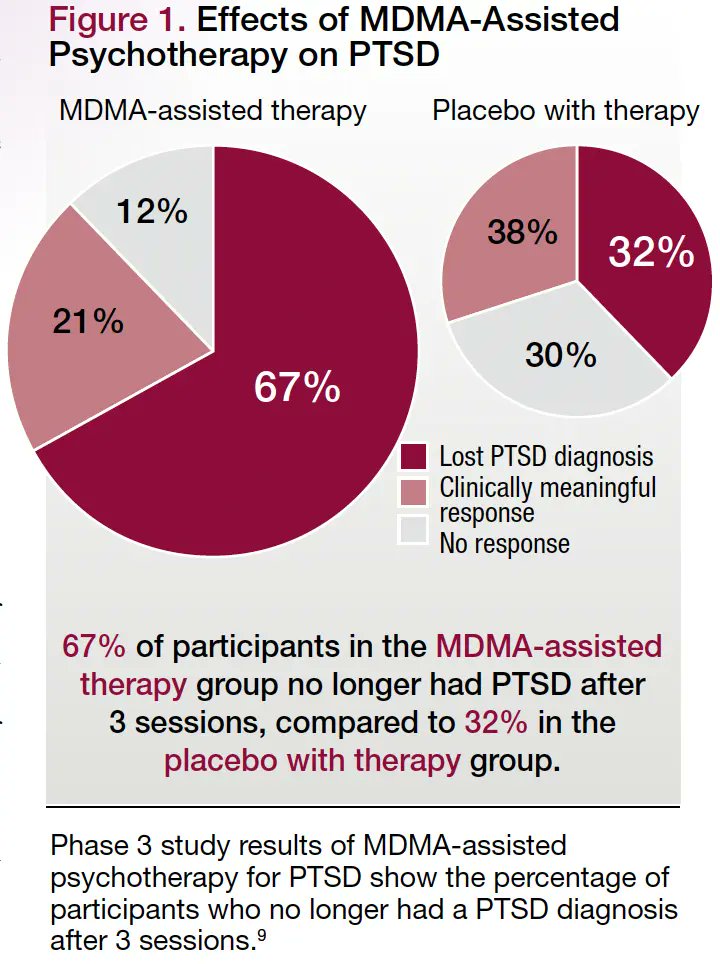
At the conclusion of the trial’s MDMA-assisted therapy course, 67% of participants no longer met the diagnostic criteria for PTSD, and more than a fifth displayed a ‘clinically significant response’. Depression symptoms plummeted, and there was no evidence of increased suicidal thinking or potential for misuse. One participant reported, “Literally, I’m a different person.”
What’s next?
The FDA will need a second successful Phase 3 trial before it approves MDMA-assisted therapy – which is now underway with 100 participants. FDA approval could be granted as soon as 2023.
Mental health experts and patients alike hope this research and eventual approval is only the start. Other studies reveal MDMA’s potential in assisting difficult-to-treat mental health issues like alcohol abuse, OCD, phobias, eating disorders, depression, end-of-life anxiety, social anxiety in autistic adults.
After that, the sky is the limit. Other psychedelics are being investigated with renewed vigour for their therapeutic potential. Psilocybin (the psychedelic component in ‘magic mushrooms’) was recently found to be more effective than a common antidepressant.
Jennifer Mitchell, lead author of the MDMA study, is thrilled, “this is a wonderful, fruitful time for discovery, because people are suddenly willing to consider these substances as therapeutics again, which hasn’t happened in 50 years”.
Cover photo by Possessed Photography on Unsplash.
Follow Maddie’s journalism journey on Twitter.
Sign Up To Our Free Newsletter