The Australian Therapeutic Goods Association (TGA) has provisionally approved the Pfizer COVID vaccine for children aged 5-11 year olds. This means the vaccine will likely be available for children before the 2022 school year begins.
Provisional approval is the most important step in the authorisation process – it means the TGA has declared the vaccine both safe and effective against COVID for children aged 5-11. The Moderna vaccine is currently also under consideration, with TGA expected to announce a decision within weeks.
The government is hoping the rollout in this age group will follow trends in the 12-15 year-old bracket. Uptake in the latter age group has been fast, with more than 76% of 12-15 year olds having received their first dose. The date for the rollout to begin has been pencilled as January 10th.
Pfizer published trials of their vaccine for 5-11 year olds in early November, reporting over 90% efficacy. But federal Health Minister Greg Hunt said the trial had too small of a participant pool at 2,268. Australia’s top immunisation advisers chose to wait on further data to eventuate from the US, which approved rollout in the age group on October 29th.
What’s different about this rollout?
Pfizer doses for 5-11 year olds will only be one third of adult doses. That is, each jab will be precisely 10 milligrams instead of the standard 30 milligrams. In order to avoid mix-ups, children’s vials will have orange caps, while adult doses come with grey or purple caps.
ATAGI will also be considering expanding the interval between doses from 3 weeks to 2 months for children. TGA chief Professor John Skerritt commented, “There’s been some emerging decisions in places like Canada that are suggesting that children should be done two months apart, or eight weeks apart, to get a stronger immune response.”
What have other countries done?
The USA has been rolling out Pfizer in 5-11 year olds for over a month now, with 10% of eligible children having had their first dose. Canada has also recently approved Pfizer for the age group, and started their rollout last week.
The European Medicines Agency has recommended Pfizer be given to 5-11 year old children. Italy approved the measure last week, and Germany is expected to follow suit early next year. In France, high-risk children or those that live with vulnerable individuals are able to get vaccinated.
Israel, Oman and Saudi Arabia have all approved vaccination for over-5 year-olds.
Are there any risks?
Some parents are hesitant about vaccinating their children due to an apparent increased risk of myocarditis. But this side effect is extremely rare. The highest figures suggest less than 200 cases per million in fully vaccinated 12-15 year-old males, and only 30 per million for females.
The risk is expected to be even smaller for younger children, as myocarditis from any cause is less common in younger ages. The lower vaccine dose reduces the risk even further.
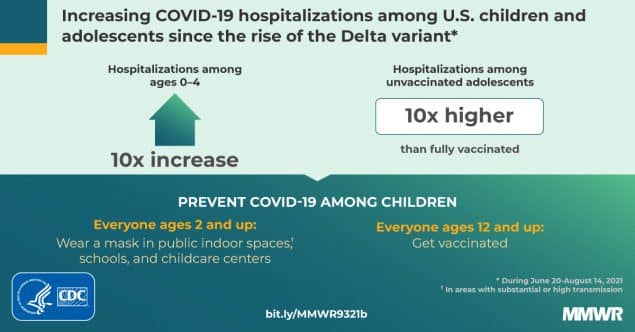
Kids are at much higher risk of contracting COVID, which can result in severe sickness and side effects like Long COVID. COVID can also trigger Multisystem Inflammatory System in children (MIS-C), which is sometimes deadly. MIS-C is most common in 5-11 year olds.
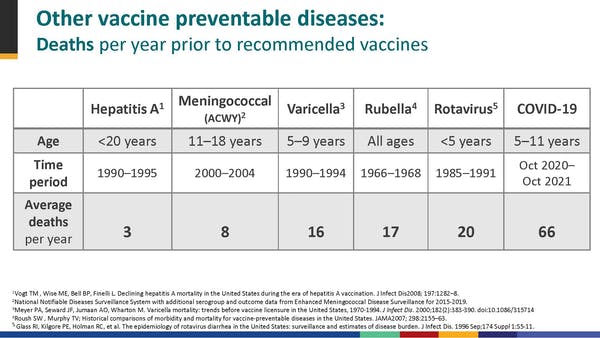
The argument for vaccinating comes down to the fact that no child should die from a vaccine-preventable disease. COVID was the 8th highest killer of 5-11 year olds over the past year.
Follow Maddie’s journalism journey on Twitter.
Sign Up To Our Free Newsletter